Parkinson-Krankheit: Neue Erkenntnisse über ein reisendes Protein
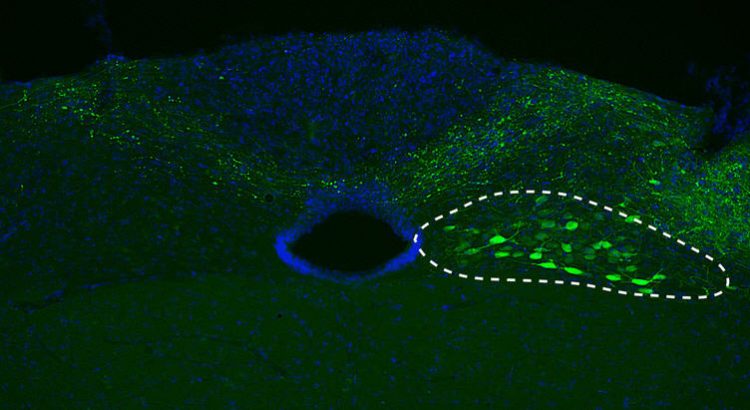
Für die aktuelle Studie schleusten DZNE-Forscher den Bauplan der menschlichen Variante von Alpha-Synuclein in die Nervenzellen (siehe gestrichelte Linie) von Mäusen. Quelle: DZNE/M. Helwig
In Parkinson’s disease, the protein “alpha-synuclein” aggregates within neurons of patients and appears to propagate across interconnected areas of the brain.
How this happens remains largely unknown. It has been proposed that alpha-synuclein may behave like a “prion”: pathological forms of the protein may be capable of changing the conformation of normal alpha-synuclein and thus triggering its aggregation and neuron-to-neuron propagation (a phenomenon referred to as “seeding”).
Recent findings by scientists at the German Center for Neurodegenerative Diseases (DZNE) reveal that aggregation, spreading and pathology caused by alpha-synuclein do not necessarily involve prion-like seeding. Instead, they could be triggered by enhanced alpha-synuclein expression and trans-neuronal passage of monomeric and oligomeric forms of the protein. Researchers led by Prof. Donato Di Monte report on this in the journal “BRAIN”.
Abundant evidence underscores a critical role of the protein alpha-synuclein in the pathogenesis of Parkinson’s disease. In particular, alpha-synuclein is a major component of the intraneuronal inclusions, named Lewy bodies, that are progressively accumulated in the brains of patients with Parkinson’s disease.
Alpha-synuclein pathology often starts in a region of the lower brain called medulla oblongata from where it spreads upwardly toward midbrain and cortical areas. In the current study, sponsored in part by the Paul Foundation, DZNE researchers mimicked this phenomenon in mice. With the aid of a tailor-made viral vector, they transferred the blueprint of the human alpha-synuclein gene specifically into neurons in the mouse medulla oblongata. These cells then began producing and accumulating relatively large amounts of the exogenous (human) alpha-synuclein.
Long-distance protein transmission
Using specific antibodies that recognize human alpha-synuclein, Di Monte and his colleagues tracked the spreading of this protein throughout the mouse brain over a period of 6 to 12 weeks. They also compared spreading and pathology in normal mice, which expressed both exogenous (human) and endogenous alpha-synuclein, versus mutant mice lacking their endogenous protein.
In both groups of animals, increased expression of human alpha-synuclein resulted in its progressive diffusion from the medulla oblongata toward more rostral brain regions. This protein spreading involved at least one trans-synaptic jump and followed a stereotypical pattern consistent with diffusion via anatomically interconnected pathways. Furthermore, accumulation of the spreading protein within recipient neurons was accompanied by evidence of neuronal damage.
Unlike prions
A prion-like seeding mechanism would predict that spreading of alpha-synuclein should be facilitated by interactions between abnormal forms of the protein generated within donor neurons and “uncorrupted” alpha-synuclein expressed within recipient cells. “In other words,” says Di Monte “we were expecting less efficient protein transmission and less pronounced pathology in mutant mice lacking endogenous alpha-synuclein. We were also expecting spreading and pathology to be associated with the accumulation of amyloidogenic alpha-synuclein; these are forms of the protein capable of producing insoluble fibrous aggregates.”
Contrary to these predictions, spreading of alpha-synuclein was enhanced rather than being counteracted by ablation of the endogenous protein in mutant mice. Furthermore, trans-neuronal passage of non-fibrillar alpha-synuclein species was responsible for protein diffusion and triggered neuronal pathology. The researcher explains, “We believe that these findings bear a number of important implications for disease pathogenesis. Not only can we conclude that long-distance diffusion of alpha-synuclein does not necessarily require the generation of prion-like species. Our data also reveal that spreading and pathology can be triggered by simple overexpression of the protein and are mediated, at least initially, by monomeric and/or oligomeric alpha-synuclein.”
Moving forward with studies on a “moving” protein
The possibility that alpha-synuclein may behave like a prion has raised the speculation that, similar to some prion diseases (for example, Creutzfeldt-Jakob disease), cases of Parkinson’s disease may arise from exposure to contagious protein species. Di Monte stresses: “There is absolutely no indication that Parkinson’s could be a contagious disease. In fact, an important contribution of our new study is that it emphasizes how critical aspects of Parkinson’s disease pathogenesis, such as neuron-to-neuron alpha-synuclein transmission and protein aggregation, can be explained by mechanisms that are not prion-like.”
Di Monte and his colleagues at the DZNE intend to continue working on alpha-synuclein and are particularly interested in elucidating how alpha-synuclein could be targeted to slow down or halt the pathologic and clinical progression of the disease.
Original publication
„Brain propagation of transduced α-synuclein involves nonfibrillar protein species and is enhanced in α-synuclein null mice“, Michael Helwig, Michael Klinkenberg, Raffaella Rusconi, Ruth E. Musgrove, Nour K. Majbour, Omar M.A. El-Agnaf, Ayse Ulusoy and Donato A. Di Monte, BRAIN, DOI: 10.1093/brain/awv376
The German Center for Neurodegenerative Diseases (DZNE) investigates the causes of diseases of the nervous system and develops strategies for prevention, treatment and care. It is an institution within the Helmholtz Association of German Research Centres with nine sites across Germany (Berlin, Bonn, Dresden, Göttingen, Magdeburg, Munich, Rostock/Greifswald, Tübingen and Witten). The DZNE cooperates closely with universities, their clinics and other research facilities.
Web: www.dzne.de/en | Twitter: @dzne_en | Facebook: www.dzne.de/facebook
http://www.dzne.de/en/about-us/public-relations/meldungen/2016/press-release-no-…
Media Contact
Weitere Informationen:
http://www.dzne.deAlle Nachrichten aus der Kategorie: Biowissenschaften Chemie
Der innovations-report bietet im Bereich der "Life Sciences" Berichte und Artikel über Anwendungen und wissenschaftliche Erkenntnisse der modernen Biologie, der Chemie und der Humanmedizin.
Unter anderem finden Sie Wissenswertes aus den Teilbereichen: Bakteriologie, Biochemie, Bionik, Bioinformatik, Biophysik, Biotechnologie, Genetik, Geobotanik, Humanbiologie, Meeresbiologie, Mikrobiologie, Molekularbiologie, Zellbiologie, Zoologie, Bioanorganische Chemie, Mikrochemie und Umweltchemie.
Neueste Beiträge

Diamantstaub leuchtet hell in Magnetresonanztomographie
Mögliche Alternative zum weit verbreiteten Kontrastmittel Gadolinium. Eine unerwartete Entdeckung machte eine Wissenschaftlerin des Max-Planck-Instituts für Intelligente Systeme in Stuttgart: Nanometerkleine Diamantpartikel, die eigentlich für einen ganz anderen Zweck bestimmt…
Neue Spule für 7-Tesla MRT | Kopf und Hals gleichzeitig darstellen
Die Magnetresonanztomographie (MRT) ermöglicht detaillierte Einblicke in den Körper. Vor allem die Ultrahochfeld-Bildgebung mit Magnetfeldstärken von 7 Tesla und höher macht feinste anatomische Strukturen und funktionelle Prozesse sichtbar. Doch alleine…

Hybrid-Energiespeichersystem für moderne Energienetze
Projekt HyFlow: Leistungsfähiges, nachhaltiges und kostengünstiges Hybrid-Energiespeichersystem für moderne Energienetze. In drei Jahren Forschungsarbeit hat das Konsortium des EU-Projekts HyFlow ein extrem leistungsfähiges, nachhaltiges und kostengünstiges Hybrid-Energiespeichersystem entwickelt, das einen…